Search results
Search for "carbon disulfide" in Full Text gives 40 result(s) in Beilstein Journal of Organic Chemistry.
Aldiminium and 1,2,3-triazolium dithiocarboxylate zwitterions derived from cyclic (alkyl)(amino) and mesoionic carbenes
- Nedra Touj,
- François Mazars,
- Guillermo Zaragoza and
- Lionel Delaude
Beilstein J. Org. Chem. 2023, 19, 1947–1956, doi:10.3762/bjoc.19.145
- cyclic (alkyl)(amino) or mesoionic carbenes (CAACs or MICs) onto carbon disulfide. Nine novel compounds were isolated and fully characterized by 1H and 13C NMR, FTIR, and HRMS techniques. Moreover, the molecular structures of two CAAC·CS2 and two MIC·CS2 betaines were determined by X-ray diffraction
- applications [24][25][26][27][28]. N-Heterocyclic carbenes and their enetetramine dimers readily add to the central carbon atom of allenes and heteroallenes X=C=Y (X, Y = CR2, NR, O, S) to afford zwitterionic adducts [29]. In particular, their reaction with carbon disulfide affords stable azolium-2
- (−)-menthone was obtained in a separate step by deprotonating the corresponding aldiminium triflate with lithium diisopropylamide (LDA) at −78 °C [8]. Herein, we disclose the synthesis of three CAAC·CS2 and six MIC·CS2 inner salts from the corresponding aldiminium or 1,2,3-triazolium salts and carbon disulfide

Graphical Abstract

Figure 1: Various types of stable singlet carbenes and their acronyms.

Figure 2: Various types of NHC·CS2 zwitterions and their coordination modes.

Scheme 1: Synthesis of CAAC·CS2 zwitterion 2 from its free carbene parent 1.

Scheme 2: Synthesis of CAAC·CS2 zwitterions 4a–c with KN(SiMe3)2.

Scheme 3: Synthesis of 1,2,3-triazolium iodides 5a–f.

Scheme 4: Synthesis of MIC·CS2 zwitterions 6a and 6b with KN(SiMe3)2.

Scheme 5: Synthesis of MIC·CS2 zwitterions 6c–f with NaOt-Bu.

Figure 3: ORTEP representations of zwitterions 4a (CAAC-Mes-Cy·CS2, top) and 4c (CAAC-Die-MePh·CS2, bottom) w...

Figure 4: ORTEP representations of zwitterions 6b (MIC-Dip-Ph-Me·CS2, top) and 6e (MIC-Mes-Bu-Me·CS2, bottom)...
Cholyl 1,3,4-oxadiazole hybrid compounds: design, synthesis and antimicrobial assessment
- Anas J. Rasras,
- Mohamed El-Naggar,
- Nesreen A. Safwat and
- Raed A. Al-Qawasmeh
Beilstein J. Org. Chem. 2022, 18, 631–638, doi:10.3762/bjoc.18.63
- available cholic acid, which was converted to its cholyl hydrazide (1) as previously reported by us [21]. The produced cholyl hydrazide 1 was heterocyclized to 1,3,4-oxadiazole-2-thiol 2 in excellent yield (93%), via the treatment with carbon disulfide and trimethylamine in refluxing ethanol (Scheme 1) [33

Graphical Abstract

Figure 1: Biologically active cholic acid hybridized with different heterocyclic scaffolds.

Scheme 1: Synthesis of cholyl 1,3,4-oxadiazole-2-thiol 2.

Scheme 2: Synthesis of cholyl 2-(propargylthio)-1,3,4-oxadiazole 3.

Scheme 3: Synthesis of target compounds 4a–v.

Figure 2: Structures of target compounds 4a–v.
Double-headed nucleosides: Synthesis and applications
- Vineet Verma,
- Jyotirmoy Maity,
- Vipin K. Maikhuri,
- Ritika Sharma,
- Himal K. Ganguly and
- Ashok K. Prasad
Beilstein J. Org. Chem. 2021, 17, 1392–1439, doi:10.3762/bjoc.17.98

Graphical Abstract

Figure 1: Double-headed nucleosides. B1 and B2 = nucleobases or heterocyclic/carbocyclic moieties; L = linker....

Scheme 1: Synthesis of 2′-(pyrimidin-1-yl)methyl- or 2′-(purin-9-yl)methyl-substituted double-headed nucleosi...

Scheme 2: Synthesis of double-headed nucleoside 7 having two cytosine moieties.

Scheme 3: Synthesis of double-headed nucleoside 2′-deoxy-2′-C-(2-(thymine-1-yl)ethyl)-uridine (11).

Scheme 4: Double-headed nucleosides 14 and 15 obtained by click reaction.

Scheme 5: Synthesis of the double-headed nucleoside 19.

Scheme 6: Synthesis of the double-headed nucleosides 24 and 25.

Scheme 7: Synthesis of double-headed nucleosides 28 and 29.

Scheme 8: Synthesis of double-headed nucleoside 33.

Scheme 9: Synthesis of double-headed nucleoside 37.

Scheme 10: Synthesis of the double-headed nucleoside 1-(5′-O-(4,4′-dimethoxytrityl)-2′-C-((4-(pyren-1-yl)-1,2,...

Scheme 11: Synthesis of triazole-containing double-headed ribonucleosides 46a–c and 50a–e.

Scheme 12: Synthesis of double-headed nucleosides 54a–g.

Scheme 13: Synthesis of double-headed nucleosides 59 and 60.

Scheme 14: Synthesis of the double-headed nucleosides 63 and 64.

Scheme 15: Synthesis of double-headed nucleosides 66a–c.

Scheme 16: Synthesis of benzoxazole-containing double-headed nucleosides 69 and 71 from 5′-amino-5′-deoxynucle...

Scheme 17: Synthesis of 4′-C-((N6-benzoyladenin-9-yl)methyl)thymidine (75) and 4′-C-((thymin-1-yl)methyl)thymi...

Scheme 18: Synthesis of double-headed nucleosides 5′-(adenine-9-yl)-5′-deoxythymidine (79) and 5′-(adenine-9-y...

Scheme 19: Synthesis of double-headed nucleosides 85–87 via reversed nucleosides methodology.

Scheme 20: Double-headed nucleosides 91 and 92 derived from ω-terminal-acetylenic sugar derivatives 90a,b.

Scheme 21: Synthesis of double-headed nucleosides 96a–g.

Scheme 22: Synthesis of double-headed nucleosides 100 and 103.

Scheme 23: Double-headed nucleosides 104 and 105 with a triazole motif.

Scheme 24: Synthesis of the double-headed nucleosides 107 and 108.

Scheme 25: Synthesis of double-headed nucleoside 110 with additional nucleobase in 5′-(S)-C-position joined th...

Scheme 26: Synthesis of double-headed nucleosides 111–113 with additional nucleobases in the 5′-(S)-C-position...

Scheme 27: Synthesis of double-headed nucleoside 114 by click reaction.

Scheme 28: Synthesis of double-headed nucleosides 118 with an additional nucleobase at the 5′-(S)-C-position.

Scheme 29: Synthesis of bicyclic double-headed nucleoside 122.

Scheme 30: Synthesis of double-headed nucleosides 125a–c derived from 2′-amino-LNA.

Scheme 31: Double-headed nucleoside 127 obtained by click reaction.

Scheme 32: Synthesis of double-headed nucleoside 130.

Scheme 33: Double-headed nucleosides 132a–d and 134a–d synthesized by Sonogashira cross coupling reaction.

Scheme 34: Synthesis of double-headed nucleosides 137 and 138 via Suzuki coupling.

Scheme 35: Synthesis of double-headed nucleosides 140 and 141 via Sonogashira cross coupling reaction.

Scheme 36: Synthesis of double-headed nucleoside 143.

Scheme 37: Synthesis of the double-headed nucleoside 146.

Scheme 38: Synthesis of 5-C-alkynyl-functionalized double-headed nucleosides 151a–d.

Scheme 39: Synthesis of 5-C-triazolyl-functionalized double-headed nucleosides 154a, b.

Scheme 40: Synthesis of double-headed nucleosides 157a–c.

Scheme 41: Synthesis of double-headed nucleoside 159, phosphoramidite 160 and the corresponding nucleotide mon...

Scheme 42: Synthesis of double-headed nucleoside 163, phosphoramidite 164 and the corresponding nucleotide mon...

Scheme 43: Synthesis of double-headed nucleoside 167, phosphoramidite 168, and the corresponding nucleotide mo...

Scheme 44: Synthesis of double-headed nucleoside 171, phosphoramidite 172, and the corresponding nucleotide mo...

Scheme 45: Synthesis of double-headed nucleoside 175, phosphoramidite 176, and the corresponding nucleotide mo...

Scheme 46: Synthesis of double-headed nucleoside 178.

Scheme 47: Synthesis of the double-headed nucleosides 181 and 183.

Scheme 48: Alternative synthesis of the double-headed nucleoside 183.

Scheme 49: Synthesis of double-headed nucleoside 188 through thermal [2 + 3] sydnone–alkyne cycloaddition reac...

Scheme 50: Synthesis of the double-headed nucleosides 190 and 191.

Scheme 51: Synthesis of 1-((5S)-2,3,4-tri-O-acetyl-5-(2,6-dichloropurin-9-yl)-β-ᴅ-xylopyranosyl)uracil (195).

Scheme 52: Synthesis of hexopyranosyl double-headed pyrimidine homonucleosides 200a–c.

Figure 2: 3′-C-Ethynyl-β-ᴅ-allopyranonucleoside derivatives 201a–f.

Scheme 53: Synthesis of 3′-C-(1,4-disubstituted-1,2,3-triazolyl)-double-headed pyranonucleosides 203–207.

Scheme 54: Synthesis of 3′-C-(1,4-disubstituted-1,2,3-triazolyl)-double-headed pyranonucleosides 208 and 209.

Scheme 55: Synthesis of 3′-C-(1,4-disubstituted-1,2,3-triazolyl)-double-headed pyranonucleoside 210.

Scheme 56: Synthesis of double-headed acyclic nucleosides (2S,3R)-1,4-bis(thymine-1-yl)butane-2,3-diol (213a) ...

Scheme 57: Synthesis of double-headed acyclic nucleosides (2R,3S)-1,4-bis(thymine-1-yl)butane-2,3-diol (213c) ...

Scheme 58: Synthesis of double-headed acetylated 1,3,4-oxadiazino[6,5-b]indolium-substituted C-nucleosides 218b...

Scheme 59: Synthesis of double-headed acyclic nucleoside 222.

Scheme 60: Synthesis of functionalized 1,2-bis(1,2,4-triazol-3-yl)ethane-1,2-diols 223a–f.

Scheme 61: Synthesis of acyclic double-headed 1,2,4-triazino[5,6-b]indole C-nucleosides 226–231.

Scheme 62: Synthesis of double-headed 1,3,4-thiadiazoline, 1,3,4-oxadiazoline, and 1,2,4-triazoline acyclo C-n...

Scheme 63: Synthesis of double-headed acyclo C-nucleosides 240–242.

Scheme 64: Synthesis of double-headed acyclo C-nucleoside 246.

Scheme 65: Synthesis of acyclo double-headed nucleoside 250.

Scheme 66: Synthesis of acyclo double-headed nucleoside 253.

Scheme 67: Synthesis of acyclo double-headed nucleosides 259a–d.

Scheme 68: Synthesis of acyclo double-headed nucleoside 261.
Synthetic reactions driven by electron-donor–acceptor (EDA) complexes
- Zhonglie Yang,
- Yutong Liu,
- Kun Cao,
- Xiaobin Zhang,
- Hezhong Jiang and
- Jiahong Li
Beilstein J. Org. Chem. 2021, 17, 771–799, doi:10.3762/bjoc.17.67
- step without any transition-metal catalyst, ligand, or photocatalyst, this method possesses a splendid application prospect. The reaction mechanism is as follows (Scheme 48): Firstly, carbon disulfide combines with N-methylaniline (134) in the presence of Cs2CO3 to form thiolate 136. Thiolate 136 is

Graphical Abstract

Scheme 1: The electron transfer process in EDA complexes.

Scheme 2: Synthesis of benzo[b]phosphorus oxide 3 initiated by an EDA complex.

Scheme 3: Mechanism of the synthesis of quinoxaline derivative 7.

Scheme 4: Synthesis of imidazole derivative 10 initiated by an EDA complex.

Scheme 5: Synthesis of sulfamoylation product 12 initiated by an EDA complex.

Scheme 6: Mechanism of the synthesis of sulfamoylation product 12.

Scheme 7: Synthesis of indole derivative 22 initiated by an EDA complex.

Scheme 8: Synthesis of perfluoroalkylated pyrimidines 26 initiated by an EDA complex.

Scheme 9: Synthesis of phenanthridine derivative 29 initiated by an EDA complex.

Scheme 10: Synthesis of cis-tetrahydroquinoline derivative 32 initiated by an EDA complex.

Scheme 11: Mechanism of the synthesis of cis-tetrahydroquinoline derivative 32.

Scheme 12: Synthesis of phenanthridine derivative 38 initiated by an EDA complex.

Scheme 13: Synthesis of spiropyrroline derivative 40 initiated by an EDA complex.

Scheme 14: Synthesis of benzothiazole derivative 43 initiated by an EDA complex.

Scheme 15: Synthesis of perfluoroalkyl-s-triazine derivative 45 initiated by an EDA complex.

Scheme 16: Synthesis of indoline derivative 47 initiated by an EDA complex.

Scheme 17: Mechanism of the synthesis of spirocyclic indoline derivative 47.

Scheme 18: Synthesis of cyclobutane product 50 initiated by an EDA complex.

Scheme 19: Mechanism of the synthesis of spirocyclic indoline derivative 50.

Scheme 20: Synthesis of 1,3-oxazolidine compound 59 initiated by an EDA complex.

Scheme 21: Synthesis of trifluoromethylated product 61 initiated by an EDA complex.

Scheme 22: Synthesis of indole alkylation product 64 initiated by an EDA complex.

Scheme 23: Synthesis of perfluoroalkylation product 67 initiated by an EDA complex.

Scheme 24: Synthesis of hydrotrifluoromethylated product 70 initiated by an EDA complex.

Scheme 25: Synthesis of β-trifluoromethylated alkyne product 71 initiated by an EDA complex.

Scheme 26: Mechanism of the synthesis of 2-phenylthiophene derivative 74.

Scheme 27: Synthesis of allylated product 80 initiated by an EDA complex.

Scheme 28: Synthesis of trifluoromethyl-substituted alkynyl product 84 initiated by an EDA complex.

Scheme 29: Synthesis of dearomatized fluoroalkylation product 86 initiated by an EDA complex.

Scheme 30: Mechanism of the synthesis of dearomatized fluoroalkylation product 86.

Scheme 31: Synthesis of C(sp3)–H allylation product 91 initiated by an EDA complex.

Scheme 32: Synthesis of perfluoroalkylation product 93 initiated by an EDA complex.

Scheme 33: Synthesis of spirocyclic indolene derivative 95 initiated by an EDA complex.

Scheme 34: Synthesis of perfluoroalkylation product 97 initiated by an EDA complex.

Scheme 35: Synthesis of alkylated indole derivative 100 initiated by an EDA complex.

Scheme 36: Mechanism of the synthesis of alkylated indole derivative 100.

Scheme 37: Synthesis of arylated oxidized indole derivative 108 initiated by an EDA complex.

Scheme 38: Synthesis of 4-ketoaldehyde derivative 111 initiated by an EDA complex.

Scheme 39: Mechanism of the synthesis of 4-ketoaldehyde derivative 111.

Scheme 40: Synthesis of perfluoroalkylated olefin 118 initiated by an EDA complex.

Scheme 41: Synthesis of alkylation product 121 initiated by an EDA complex.

Scheme 42: Synthesis of acylation product 123 initiated by an EDA complex.

Scheme 43: Mechanism of the synthesis of acylation product 123.

Scheme 44: Synthesis of trifluoromethylation product 126 initiated by an EDA complex.

Scheme 45: Synthesis of unnatural α-amino acid 129 initiated by an EDA complex.

Scheme 46: Synthesis of thioether derivative 132 initiated by an EDA complex.

Scheme 47: Synthesis of S-aryl dithiocarbamate product 135 initiated by an EDA complex.

Scheme 48: Mechanism of the synthesis of S-aryl dithiocarbamate product 135.

Scheme 49: Synthesis of thioether product 141 initiated by an EDA complex.

Scheme 50: Mechanism of the synthesis of borate product 144.

Scheme 51: Synthesis of boronation product 148 initiated by an EDA complex.

Scheme 52: Synthesis of boration product 151 initiated by an EDA complex.

Scheme 53: Synthesis of boronic acid ester derivative 154 initiated by an EDA complex.

Scheme 54: Synthesis of β-azide product 157 initiated by an EDA complex.

Scheme 55: Decarboxylation reaction initiated by an EDA complex.

Scheme 56: Synthesis of amidated product 162 initiated by an EDA complex.

Scheme 57: Synthesis of diethyl phenylphosphonate 165 initiated by an EDA complex.

Scheme 58: Mechanism of the synthesis of diethyl phenylphosphonate derivative 165.

Scheme 59: Synthesis of (Z)-2-iodovinyl phenyl ether 168 initiated by an EDA complex.

Scheme 60: Mechanism of the synthesis of (Z)-2-iodovinyl phenyl ether derivative 168.

Scheme 61: Dehalogenation reaction initiated by an EDA complex.
[2 + 1] Cycloaddition reactions of fullerene C60 based on diazo compounds
- Yuliya N. Biglova
Beilstein J. Org. Chem. 2021, 17, 630–670, doi:10.3762/bjoc.17.55
- soluble in the majority of organic solvents and only partially soluble in carbon disulfide, which is expected to ultimately lead to similar insoluble hard-to-recover polymers. It is believed [95] that the best solution to the problem seems to involve the incorporation of solubilizing groups into the
Valorisation of plastic waste via metal-catalysed depolymerisation
- Francesca Liguori,
- Carmen Moreno-Marrodán and
- Pierluigi Barbaro
Beilstein J. Org. Chem. 2021, 17, 589–621, doi:10.3762/bjoc.17.53

- Polystyrene (PS): PS is a low-cost, hard and brittle plastic used both as a solid or foam in protective packaging, containers and trays [151]. It is a nonbiodegradable material accounting for about 10% of municipal solid waste [135]. It is soluble in benzene, carbon disulfide, chlorinated hydrocarbons, lower

Graphical Abstract

Figure 1: Potential classification of plastic recycling processes. The area covered by the present review is ...

Figure 2: EG produced during glycolytic depolymerisation of PET using DEG + DPG as solvent and titanium(IV) n...

Scheme 1: Simplified representation of the conversion of 1,4-PBD to C16–C44 macrocycles using Ru metathesis c...

Figure 3: Main added-value monomers obtainable by catalytic depolymerisation of PET via chemolytic methods.

Scheme 2: Hydrogenolytic depolymerisation of PET by ruthenium complexes.

Scheme 3: Depolymerisation of PET via catalytic hydrosilylation by Ir(III) pincer complex.

Scheme 4: Catalytic hydrolysis (top) and methanolysis (bottom) reactions of PET.

Scheme 5: Depolymerisation of PET by glycolysis with ethylene glycol.

Figure 4: Glycolysis of PET: evolution of BHET yield over time, with and without zinc acetate catalyst (196 °...

Scheme 6: Potential activated complex for the glycolysis reaction of PET catalysed by metallated ILs and evol...

Scheme 7: One-pot, two-step process for PET repurposing via chemical recycling.

Scheme 8: Synthetic routes to PLA.

Scheme 9: Structures of the zinc molecular catalysts used for PLA-methanolysis in various works. a) See [265], b) ...

Scheme 10: Depolymerisation of PLLA by Zn–N-heterocyclic carbene complex.

Scheme 11: Salalen ligands.

Scheme 12: Catalytic hydrogenolysis of PLA.

Scheme 13: Catalytic hydrosilylation of PLA.

Scheme 14: Hydrogenative depolymerisation of PBT and PCL by molecular Ru catalysts.

Scheme 15: Glycolysis reaction of PCT by diethylene glycol.

Scheme 16: Polymerisation–depolymerisation cycle of 3,4-T6GBL.

Scheme 17: Polymerisation–depolymerisation cycle of 2,3-HDB.

Scheme 18: Hydrogenative depolymerisation of PBPAC by molecular Ru catalysts.

Scheme 19: Catalytic hydrolysis (top), alcoholysis (middle) and aminolysis (bottom) reactions of PBPAC.

Scheme 20: Hydrogenative depolymerisation of PPC (top) and PEC (bottom) by molecular Ru catalysts.

Scheme 21: Polymerisation-depolymerisation cycle of BEP.

Scheme 22: Hydrogenolysis of polyamides using soluble Ru catalysts.

Scheme 23: Catalytic depolymerisation of epoxy resin/carbon fibres composite.

Scheme 24: Depolymerisation of polyethers with metal salt catalysts and acyl chlorides.

Scheme 25: Proposed mechanism for the iron-catalysed depolymerisation reaction of polyethers. Adapted with per...
Selective and reversible 1,3-dipolar cycloaddition of 6-aryl-1,5-diazabicyclo[3.1.0]hexanes with 1,3-diphenylprop-2-en-1-ones under microwave irradiation
- Alexander P. Molchanov,
- Mariia M. Efremova,
- Mariya A. Kryukova and
- Mikhail A. Kuznetsov
Beilstein J. Org. Chem. 2020, 16, 2679–2686, doi:10.3762/bjoc.16.218
- with carbon disulfide [26], diphenylketene [27], β-nitrostyrenes [28][29], if ionic liquids (IL) were used as reaction media. The reaction of β-nitrostyrenes with azomethine imines proceeds giving mixtures of Michael and anti-Michael-type adducts, containing a nitro group at the C2- or C1-position of

Graphical Abstract

Scheme 1: The two types of azomethine imines (AMI).

Scheme 2: Reaction of 1,5-diazabicyclo[3.1.0]hexanes 1a–d with diarylpropenones 2a–l.

Figure 1: Single-crystal X-ray structure of compound 3e.

Figure 2: Single-crystal X-ray structure of compound 3g.

Scheme 3: Control experiments.

Scheme 4: Mechanistic hypothesis for cycloaddition and cycloreversion reactions of diazabicyclohexane 1a with...

Scheme 5: Experiments on the trapping of azomethine imine, generated from pyrazolopyrazole 3g.
A new method for the synthesis of diamantane by hydroisomerization of binor-S on treatment with sulfuric acid
- Rishat I. Aminov and
- Ravil I. Khusnutdinov
Beilstein J. Org. Chem. 2020, 16, 2534–2539, doi:10.3762/bjoc.16.205
- , experiment B), or in carbon disulfide (CS2, experiment C). In experiment А, the major isomer 1-D2, which is formed upon hydroisomerization of binor-S (2), contains two deuterium atoms. Two more hydrogen atoms are probably provided by cyclohexane. Unexpectedly, the reaction also gave undeuterated diamantane
- (2) acts as the hydrogen source for the isomer C14H17D3, 1-D3. Our attempt to carry out the deuteration of diamantane (1) with D2SO4 in carbon disulfide for 7 h at 20 °C was unsuccessful. Evidently, the deuterium exchange, resulting in the formation of diamantanes 1-D7 and 1-D8 containing 7 and 8
- acid (98%) in carbon disulfide or cyclohexane. It was found that both, sulfuric acid and cyclohexane can serve as the main hydrogen sources. In the presence of H2SO4 with a lower concentration (75–80%), the reaction stops at the step of formation of endo-endo-pentacyclo[7.3.1.12,5.18,10.03,7

Graphical Abstract

Scheme 1: Isomerization of 3а–с to diamantane (1). Reaction conditions: (a) CoBr2·2PPh3–BF3·OEt2, 110 °C, 12 ...

Scheme 2: Isomerization of binor-S (2) to diamantane (1).

Scheme 3: Selective synthesis of tetrahydrobinor-S (3c) from binor-S (2).

Scheme 4: Isomerization of binor-S (2) to hydrocarbons 4а and b.
Recent synthesis of thietanes
- Jiaxi Xu
Beilstein J. Org. Chem. 2020, 16, 1357–1410, doi:10.3762/bjoc.16.116
- of quadricyclane 218 with thiocarbonyl derivatives 219. With carbon disulfide, mono- and biscycloadducts 221 and 222 were formed depending on concentration, temperature, and pressure conditions [69] (Scheme 43). In the same year, Kanaoka and co-workers reported the intermolecular photo [2 + 2

Graphical Abstract

Figure 1: Examples of biologically active thietane-containing molecules.

Figure 2: The diverse methods for the synthesis of thietanes.

Scheme 1: Synthesis of 1-(thietan-2-yl)ethan-1-ol (10) from 3,5-dichloropentan-2-ol (9).

Scheme 2: Synthesis of thietanose nucleosides 2,14 from 2,2-bis(bromomethyl)propane-1,3-diol (11).

Scheme 3: Synthesis of methyl 3-vinylthietane-3-carboxylate (19).

Scheme 4: Synthesis of 1,6-thiazaspiro[3.3]heptane (24).

Scheme 5: Synthesis of 6-amino-2-thiaspiro[3.3]heptane hydrochloride (28).

Scheme 6: Synthesis of optically active thietane 31 from vitamin C.

Scheme 7: Synthesis of an optically active thietane nucleoside from diethyl L-tartrate (32).

Scheme 8: Synthesis of thietane-containing spironucleoside 40 from 5-aldo-3-O-benzyl-1,2-O-isopropylidene-α-D...

Scheme 9: Synthesis of optically active 2-methylthietane-containing spironucleoside 43.

Scheme 10: Synthesis of a double-linked thietane-containing spironucleoside 48.

Scheme 11: Synthesis of two diastereomeric thietanose nucleosides via 2,4-di(benzyloxymethyl)thietane (49).

Scheme 12: Synthesis of the thietane-containing PI3k inhibitor candidate 54.

Scheme 13: Synthesis of the spirothietane 57 as the key intermediate to Nuphar sesquiterpene thioalkaloids.

Scheme 14: Synthesis of spirothietane 61 through a direct cyclic thioetherification of 3-mercaptopropan-1-ol.

Scheme 15: Synthesis of thietanes 66 from 1,3-diols 62.

Scheme 16: Synthesis of thietanylbenzimidazolone 75 from (iodomethyl)thiazolobenzimidazole 70.

Scheme 17: Synthesis of 2-oxa-6-thiaspiro[3.3]heptane (80) from bis(chloromethyl)oxetane 76 and thiourea.

Scheme 18: Synthesis of the thietane-containing glycoside, 2-O-p-toluenesulfonyl-4,6-thioanhydro-α-D-gulopyran...

Scheme 19: Synthesis of methyl 4,6-thioanhydro-α-D-glucopyranoside (89).

Scheme 20: Synthesis of thietane-fused α-D-galactopyranoside 93.

Scheme 21: Synthesis of thietane-fused α-D-gulopyranoside 100.

Scheme 22: Synthesis of 3,5-anhydro-3-thiopentofuranosides 104.

Scheme 23: Synthesis of anhydro-thiohexofuranosides 110, 112 and 113 from from 1,2:4,5-di-O-isopropylidene D-f...

Scheme 24: Synthesis of optically active thietanose nucleosides from D- and L-xyloses.

Scheme 25: Synthesis of thietane-fused nucleosides.

Scheme 26: Synthesis of 3,5-anhydro-3-thiopentofuranosides.

Scheme 27: Synthesis of 2-amino-3,5-anhydro-3-thiofuranoside 141.

Scheme 28: Synthesis of thietane-3-ols 145 from (1-chloromethyl)oxiranes 142 and hydrogen sulfide.

Scheme 29: Synthesis of thietane-3-ol 145a from chloromethyloxirane (142a).

Scheme 30: Synthesis of thietane-3-ols 145 from 2-(1-haloalkyl)oxiranes 142 and 147 with ammonium monothiocarb...

Scheme 31: Synthesis of 7-deoxy-5(20)thiapaclitaxel 154a, a thietane derivative of taxoids.

Scheme 32: Synthesis of 5(20)-thiadocetaxel 158 from 10-deacetylbaccatin III (155).

Scheme 33: Synthesis of thietane derivatives 162 as precursors for deoxythiataxoid synthesis through oxiraneme...

Scheme 34: Synthesis of 7-deoxy 5(20)-thiadocetaxel 154b.

Scheme 35: Mechanism for the formation of the thietane ring in 171 from oxiranes with vicinal leaving groups 1...

Scheme 36: Synthesis of cis-2,3-disubstituted thietane 175 from thiirane-2-methanol 172.

Scheme 37: Synthesis of a bridged thietane 183 from aziridine cyclohexyl tosylate 179 and ammonium tetrathiomo...

Scheme 38: Synthesis of thietanes via the photochemical [2 + 2] cycloaddition of thiobenzophenone 184a with va...

Scheme 39: Synthesis of spirothietanes through the photo [2 + 2] cycloaddition of cyclic thiocarbonyls with ol...

Scheme 40: Photochemical synthesis of spirothietane-thioxanthenes 210 from thioxanthenethione (208) and butatr...

Scheme 41: Synthesis of thietanes 213 from 2,4,6-tri(tert-butyl)thiobenzaldehyde (211) with substituted allene...

Scheme 42: Photochemical synthesis of spirothietanes 216 and 217 from N-methylthiophthalimide (214) with olefi...

Scheme 43: Synthesis of fused thietanes from quadricyclane with thiocarbonyl derivatives 219.

Scheme 44: Synthesis of tricyclic thietanes via the photo [2 + 2] cycloaddition of N-methyldithiosuccinimides ...

Scheme 45: Synthesis of tricyclic thietanes via the photo [2 + 2] cycloaddition of N-methylthiosuccinimide/thi...

Scheme 46: Synthesis of tricyclic thietanes via the photo [2 + 2] cycloaddition of N-alkylmonothiophthalimides...

Scheme 47: Synthesis of spirothietanes from dithiosuccinimides 223 with 2,3-dimethyl-2-butene (215a).

Scheme 48: Synthesis of thietanes 248a,b from diaryl thione 184b and ketene acetals 247a,b.

Scheme 49: Photocycloadditions of acridine-9-thiones 249 and pyridine-4(1H)-thione (250) with 2-methylacrynitr...

Scheme 50: Synthesis of thietanes via the photo [2 + 2] cycloaddition of mono-, di-, and trithiobarbiturates 2...

Scheme 51: Synthesis of spirothietanes via the photo [2 + 2] cycloaddition of 1,1,3-trimethyl-2-thioxo-1,2-dih...

Scheme 52: Synthesis of spirothietanes via the photo [2 + 2] cycloaddition of thiocoumarin 286 with olefins.

Scheme 53: Photochemical synthesis of thietanes 296–299 from semicyclic and acyclic thioimides 292–295 and 2,3...

Scheme 54: Photochemical synthesis of spirothietane 301 from 1,3,3-trimethylindoline-2-thione (300) and isobut...

Scheme 55: Synthesis of spirobenzoxazolethietanes 303 via the photo [2 + 2] cycloaddition of alkyl and aryl 2-...

Scheme 56: Synthesis of spirothietanes from tetrahydrothioxoisoquinolines 306 and 307 with olefins.

Scheme 57: Synthesis of spirothietanes from 1,3-dihydroisobenzofuran-1-thiones 311 and benzothiophene-1-thione...

Scheme 58: Synthesis of 2-triphenylsilylthietanes from phenyl triphenylsilyl thioketone (316) with electron-po...

Scheme 59: Diastereoselective synthesis of spiropyrrolidinonethietanes 320 via the photo [2 + 2] cycloaddition...

Scheme 60: Synthesis of bicyclic thietane 323 via the photo [2 + 2] cycloaddition of 2,4-dioxo-3,4-dihydropyri...

Scheme 61: Photo-induced synthesis of fused thietane-2-thiones 325 and 326 from silacyclopentadiene 324 and ca...

Scheme 62: Synthesis of highly strained tricyclic thietanes 328 via the intramolecular photo [2 + 2] cycloaddi...

Scheme 63: Synthesis of tri- and pentacyclic thietanes 330 and 332, respectively, through the intramolecular p...

Scheme 64: Synthesis of tricyclic thietanes 334 via the intramolecular photo [2 + 2] cycloaddition of N-vinylt...

Scheme 65: Synthesis of tricyclic thietanes 336 via the intramolecular photo [2 + 2] cycloaddition of N-but-3-...

Scheme 66: Synthesis of tricyclic thietanes via the intramolecular photo [2 + 2] cycloaddition of N-but-3-enyl...

Scheme 67: Synthesis of tetracyclic thietane 344 through the intramolecular photo [2 + 2] cycloaddition of N-[...

Scheme 68: Synthesis of tri- and tetracyclic thietanes 348, 350, and 351, through the intramolecular photo [2 ...

Scheme 69: Synthesis of tetracyclic fused thietane 354 via the photo [2 + 2] cycloaddition of vinyl 2-thioxo-3H...

Scheme 70: Synthesis of highly rigid thietane-fused β-lactams via the intramolecular photo [2 + 2] cycloadditi...

Scheme 71: Asymmetric synthesis of a highly rigid thietane-fused β-lactam 356a via the intramolecular photo [2...

Scheme 72: Diastereoselective synthesis of the thietane-fused β-lactams via the intramolecular photo [2 + 2] c...

Scheme 73: Asymmetric synthesis of thietane-fused β-lactams 356 via the intramolecular photo [2 + 2] cycloaddi...

Scheme 74: Synthesis of the bridged bis(trifluoromethyl)thietane from 2,2,4,4-tetrakis(trifluoromethyl)-1,3-di...

Scheme 75: Synthesis of the bridged-difluorothietane 368 from 2,2,4,4-tetrafluoro-1,3-dithietane (367) and qua...

Scheme 76: Synthesis of bis(trifluoromethyl)thietanes from 2,2,4,4-tetrakis(trifluoromethyl)-1,3-dithietane (3...

Scheme 77: Synthesis of 2,2-dimethylthio-4,4-di(trifluoromethyl)thietane (378) from 2,2,4,4-tetrakis(trifluoro...

Scheme 78: Formation of bis(trifluoromethyl)thioacetone (381) through nucleophilic attack of dithietane 363 by...

Scheme 79: Synthesis of 2,2-bis(trifluoromethyl)thietanes from 2,2,4,4-tetrakis(trifluoromethyl)-1,3-dithietan...

Scheme 80: Synthesis of the bridged bis(trifluoromethyl)thietane 364 from of 2,2,4,4-tetrakis(trifluoromethyl)...

Scheme 81: Synthesis of 2,4-diiminothietanes 390 from alkenimines and 4-methylbenzenesulfonyl isothiocyanate (...

Scheme 82: Synthesis of arylidene 2,4-diiminothietanes 393 starting from phosphonium ylides 391 and isothiocya...

Scheme 83: Synthesis of thietane-2-ylideneacetates 397 through a DABCO-catalyzed formal [2 + 2] cycloaddition ...

Scheme 84: Synthesis of 3-substituted thietanes 400 from (1-chloroalkyl)thiiranes 398.

Scheme 85: Synthesis of N-(thietane-3-yl)azaheterocycles 403 and 404 through reaction of chloromethylthiirane (...

Scheme 86: Synthesis of 3-sulfonamidothietanes 406 from sulfonamides and chloromethylthiirane (398a).

Scheme 87: Synthesis of N-(thietane-3-yl)isatins 408 from chloromethylthiirane (398a) and isatins 407.

Scheme 88: Synthesis of 3-(nitrophenyloxy)thietanes 410 from nitrophenols 409 and chloromethylthiirane (398a).

Scheme 89: Synthesis of N-aryl-N-(thietane-3-yl)cyanamides 412 from N-arylcyanamides 411 and chloromethylthiir...

Scheme 90: Synthesis of 1-(thietane-3-yl)pyrimidin-2,4(1H,3H)-diones 414 from chloromethylthiirane (398a) and ...

Scheme 91: Synthesis of 2,4-diiminothietanes 418 from 2-iminothiiranes 416 and isocyanoalkanes 415.

Scheme 92: Synthesis of 2-vinylthietanes 421 from thiiranes 419 and 3-chloroallyl lithium (420).

Scheme 93: Synthesis of thietanes from thiiranes 419 and trimethyloxosulfonium iodide 424.

Scheme 94: Mechanism for synthesis of thietanes 425 from thiiranes 419 and trimethyloxosulfonium iodide 424.

Scheme 95: Synthesis of functionalized thietanes from thiiranes and dimethylsulfonium acylmethylides.

Scheme 96: Mechanism for the rhodium-catalyzed synthesis of functionalized thietanes 429 from thiiranes 419 an...

Scheme 97: Synthesis of 3-iminothietanes 440 through thermal isomerization from 4,5-dihydro-1,3-oxazole-4-spir...

Scheme 98: Synthesis of thietanes 443 from 3-chloro-2-methylthiolane (441) through ring contraction.

Scheme 99: Synthesis of an optically active thietanose 447 from D-xylose involving a ring contraction.

Scheme 100: Synthesis of optically thietane 447 via the DAST-mediated ring contraction of 448.

Scheme 101: Synthesis of the optically thietane nucleoside 451 via the ring contraction of thiopentose in 450.

Scheme 102: Synthesis of spirothietane 456 from 3,3,5,5-tetramethylthiolane-2,4-dithione (452) and benzyne (453...

Scheme 103: Synthesis of thietanes 461 via photoisomerization of 2H,6H-thiin-3-ones 459.

Scheme 104: Phosphorodithioate-mediated synthesis of 1,4-diarylthietanes 465.

Scheme 105: Mechanism of the phosphorodithioate-mediated synthesis of 1,4-diarylthietanes 465.

Scheme 106: Phosphorodithioate-mediated synthesis of trisubstituted thietanes (±)-470.

Scheme 107: Mechanism on the phosphorodithioate-mediated synthesis of trisubstituted thietanes.

Scheme 108: Phosphorodithioate-mediated synthesis of thietanes (±)-475.

Scheme 109: Phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes from aldehydes 476 and acrylon...

Scheme 110: Phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes via a one-pot three-component ...

Scheme 111: Mechanism for the phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes via three-co...

Scheme 112: Phosphorodithioate-mediated synthesis of substituted 3-nitrothietanes.

Scheme 113: Mechanism on the phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes (±)-486.

Scheme 114: Asymmetric synthesis of (S)-2-phenylthietane (497).

Scheme 115: Asymmetric synthesis of optically active 2,4-diarylthietanes.

Scheme 116: Synthesis of 3-acetamidothietan-2-one 503 via the intramolecular thioesterification of 3-mercaptoal...

Scheme 117: Synthesis of 4-substituted thietan-2-one via the intramolecular thioesterification of 3-mercaptoalk...

Scheme 118: Synthesis of 4,4-disubstituted thietan-2-one 511 via the intramolecular thioesterification of the 3...

Scheme 119: Synthesis of a spirothietan-2-one 514 via the intramolecular thioesterification of 3-mercaptoalkano...

Scheme 120: Synthesis of thiatetrahydrolipstatin starting from (S)-(−)-epichlorohydrin ((S)-142a).

Scheme 121: Synthesis of 2-phenethyl-4-(propan-2-ylidene)thietane (520) from 5-bromo-6-methyl-1-phenylhept-5-en...

Scheme 122: Synthesis of 2-phenethyl-4-(propan-2-ylidene)thietane (520) directly from S-(5-bromo-6-methyl-1-phe...

Scheme 123: Synthesis of 2-alkylidenethietanes from S-(2-bromoalk-1-en-4-yl)thioacetates.

Scheme 124: Synthesis of 2-alkylidenethietanes from S-(2-bromo/chloroalk-1-en-4-yl)thiols.

Scheme 125: Synthesis of spirothietan-3-ol 548 from enone 545 and ammonium hydrosulfide.

Scheme 126: Asymmetric synthesis of the optically active thietanoside from cis-but-2-ene-1,4-diol (47).

Scheme 127: Synthesis of 2-alkylidenethietan-3-ols 557 via the fluoride-mediated cyclization of thioacylsilanes ...

Scheme 128: Synthesis of 2-iminothietanes via the reaction of propargylbenzene (558) and isothiocyanates 560 in...

Scheme 129: Synthesis of 2-benzylidenethietane 567 via the nickel complex-catalyzed electroreductive cyclizatio...

Scheme 130: Synthesis of 2-iminothietanes 569 via the photo-assisted electrocyclic reaction of N-monosubstitute...

Scheme 131: Synthesis of ethyl 3,4-diiminothietane-2-carboxylates from ethyl thioglycolate (570) and bis(imidoy...

Scheme 132: Synthesis of N-(thietan-3-yl)-α-oxoazaheterocycles from azaheterocyclethiones and chloromethyloxira...

Scheme 133: Synthesis of thietan-3-yl benzoate (590) via the nickel-catalyzed intramolecular reductive thiolati...

Scheme 134: Synthesis of 2,2-bis(trifluoromethyl)thietane from 3,3-bis(trifluoromethyl)-1,2-dithiolane.

Scheme 135: Synthesis of thietanes from enamines and sulfonyl chlorides.

Scheme 136: Synthesis of spirothietane 603 via the [2 + 3] cycloaddition of 2,2,4,4-tetramethylcyclobutane-1,3-...

Scheme 137: Synthesis of thietane (605) from 1-bromo-3-chloropropane and sulfur.
One-pot synthesis of dicyclopenta-fused peropyrene via a fourfold alkyne annulation
- Ji Ma,
- Yubin Fu,
- Junzhi Liu and
- Xinliang Feng
Beilstein J. Org. Chem. 2020, 16, 791–797, doi:10.3762/bjoc.16.72
- 1 were obtained by slow evaporation from a carbon disulfide solution, allowing us to disclose the molecular structure by X-ray crystallography (Figure 2a). As shown in Figure 2a, the crystal structure of 1 clearly displayed a nonplanar conformation, resulting from steric repulsion between the phenyl

Graphical Abstract

Scheme 1: Chemical structures of dicyclopenta-fused pyrene derivatives i–iii, peropyrene and the dicyclopenta...

Scheme 2: Synthetic route towards compound 1. a) B2pin2, dtbpy, [Ir(OMe)cod]2, cyclohexane, 70 °C, 20 h, 67%;...

Figure 1: High-resolution MALDI-TOF mass spectrum of 1. Inset: isotopic distribution compared to mass spectru...

Figure 2: Single-crystal X-ray structure of 1. (a) Top view and (b) side view of the (P,P) isomer. c) Crystal...

Figure 3: (a) UV–vis absorption spectra of precursor 5 and 1 in CH2Cl2 solution (10−5 M). Inset: photograph o...

Figure 4: Molecular orbitals of peropyrene derivative 6 and the dicyclopenta-fused peropyrene 1.
The use of isoxazoline and isoxazole scaffolding in the design of novel thiourea and amide liquid-crystalline compounds
- Itamar L. Gonçalves,
- Rafaela R. da Rosa,
- Vera L. Eifler-Lima and
- Aloir A. Merlo
Beilstein J. Org. Chem. 2020, 16, 175–184, doi:10.3762/bjoc.16.20
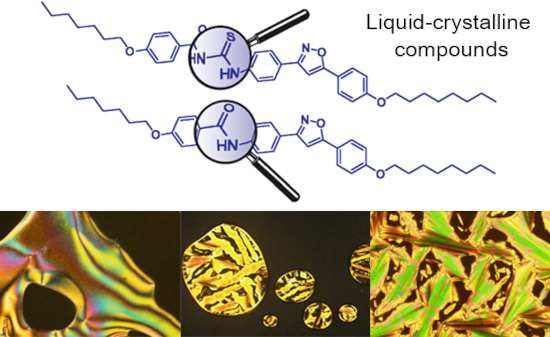
- thioureas is the reaction of carbon disulfide with either one or two different amines [9]. Due to their self-assembly and self-organization through intermolecular hydrogen bonding, thioureas display interesting technological applications to this group of molecules, one of which explores its application in

Graphical Abstract

Scheme 1: Amines 3, 4, 8, 9, 12 and 13 installed on 5-membered isoxazoline and isoxazole rings.

Scheme 2: Synthesis of acylisoxazolinylthioureas 17a–c and acylisoxazolylthioureas 18a–c. (i) SOCl2, reflux, ...

Scheme 3: Synthesis of amides. Part A: (i) SOCl2, reflux; (ii) KOCN, acetone, reflux; (iii) amines 3, 4, 8 an...

Figure 1: Optical textures observed on POM for thioureas 17a (a), 17b (b), 18a (c), 18b (d) and 18c (e,f). Al...

Figure 2: Optical textures observed on POM of amide 19. (a) Fan-shaped focal conic texture of the SmA mesopha...

Figure 3: DSC curves for the thiourea 17a (A), amide 19 (B) and 20 (C) upon the first heating and cooling cur...

Figure 4: TGA analysis for thiourea 18c; and amides 20 and 22.
Synthesis, liquid crystalline behaviour and structure–property relationships of 1,3-bis(5-substituted-1,3,4-oxadiazol-2-yl)benzenes
- Afef Mabrouki,
- Malek Fouzai,
- Armand Soldera,
- Abdelkader Kriaa and
- Ahmed Hedhli
Beilstein J. Org. Chem. 2020, 16, 149–158, doi:10.3762/bjoc.16.17

- acids in the presence of phosphorus oxychloride according to standard methods [29], oxadiazole derivatives 2 were obtained (Scheme 1). On the other hand, we converted compound 1 into sulfanyloxadiazole derivatives 4 by treatment with carbon disulfide and subsequent alkylation of the obtained

Graphical Abstract

Scheme 1: Synthesis of oxadiazole derivatives 2 and 4.

Scheme 2: Tautomeric equilibrium of compound 3.

Figure 1: DSC thermograms of fluorinated compounds 2b, 4a and 4b recorded at 5 °C/mn at heating (down traces)...

Figure 2: Optical texture (×10) of liquid crystal phase for fluorinated compounds, (a): SmA phase observed in...

Figure 3: Typical diffractogram observed for compound 2b at 398 K.

Figure 4: Typical diffractogram observed for compound 4a at 411 K.

Figure 5: Conformer of lowest energy of compounds: 4c, conformation A, (a) front view, (a’) top view, (a”) si...

Figure 6: Vector of dipole moment of compounds 4c, 4b and 2b.

Figure 7: Plot of molecular dipole moments. Orange, fluorocarbon compounds; blue, hydrocarbon compounds; gree...
A review of the total syntheses of triptolide
- Xiang Zhang,
- Zaozao Xiao and
- Hongtao Xu
Beilstein J. Org. Chem. 2019, 15, 1984–1995, doi:10.3762/bjoc.15.194
- new methodologies of butenolide formation. The first butenolide formation started with the reaction of ketone 68 with carbon disulfide (CS2) and iodomethane (MeI) to give the ketene dithioacetal intermediate 69, which was subjected to a Corey–Chaykovsky epoxidation, followed by acid hydrolysis to give

Graphical Abstract

Figure 1: Structures of triptolide (1), triptonide (2), tripdiolide (3), 16-hydroxytriptolide (4), triptrioli...

Figure 2: Syntheses of triptolide.

Scheme 1: Berchtold’s synthesis of triptolide.

Scheme 2: Li’s formal synthesis of triptolide.

Scheme 3: van Tamelen’s asymmetric synthesis of triptonide and triptolide.

Scheme 4: Van Tamelen’s (method II) formal synthesis of triptolide.

Scheme 5: Sherburn’s formal synthesis of triptolide.

Scheme 6: van Tamelen’s biogenetic type total synthesis of triptolide.

Scheme 7: Yang’s total synthesis of triptolide.

Scheme 8: Key intermediates or transformations of routes J–N.
A novel three-component reaction between isocyanides, alcohols or thiols and elemental sulfur: a mild, catalyst-free approach towards O-thiocarbamates and dithiocarbamates
- András György Németh,
- György Miklós Keserű and
- Péter Ábrányi-Balogh
Beilstein J. Org. Chem. 2019, 15, 1523–1533, doi:10.3762/bjoc.15.155
- based on the reaction of amines and the readily available, but toxic and volatile carbon disulfide [42][43][44][45]. Greener methods for the synthesis of thiocarbamates and dithiocarbamates have been developed such as the addition of the amine component to potassium thiocyanate [46][47] or

Graphical Abstract

Scheme 1: Synthetic routes to O-thiocarbamates and dithiocarbamates.

Scheme 2: Substrate scope of isocyanides. aReaction conditions: 1 (1 mmol), S8 (2 mmol), 2a (2mmol), NaH (2 m...

Scheme 3: Substrate scope of alcohols. Reaction conditions: 1a (1 mmol), S8 (2 mmol), 2 (2mmol), NaH (2 mmol)...

Scheme 4: Substrate scope of thiols. Reaction conditions: 1a (1 mmol), S8 (1.2 mmol), 4 (2 mmol), NaOH (2 mmo...

Scheme 5: Scaled-up synthesis for 3a.

Scheme 6: Multicomponent domino synthesis of quinazolinone 7.

Scheme 7: Control experiments.

Scheme 8: Proposed mechanism.
Steroid diversification by multicomponent reactions
- Leslie Reguera,
- Cecilia I. Attorresi,
- Javier A. Ramírez and
- Daniel G. Rivera
Beilstein J. Org. Chem. 2019, 15, 1236–1256, doi:10.3762/bjoc.15.121
- the 1,4-dihydropyridine scaffold. As depicted in Scheme 14, compound 46 was later subjected to a variety of post-MCR cyclizations, including the reaction with carboxylic acids to form the fused steroidal pyridopyrimidinones 47 and with carbon disulfide to form pyridopyrimidinedithione 48 in very good

Graphical Abstract

Figure 1: Structures of natural steroids of A) animal and B) plant origin.

Scheme 1: Synthesis of a steroidal β-lactam by Ugi reaction of a cholanic aldehyde [14].

Scheme 2: Synthetic route to steroidal 2,5-diketopiperazines based on a diastereoselective Ugi-4CR with an an...

Scheme 3: Multicomponent synthesis of a heterocycle–steroid hybrid using a ketosteroid as carbonyl component [18]....

Scheme 4: Synthesis of peptidomimetic–steroid hybrids using the Ugi-4CR with spirostanic amines and carboxyli...

Scheme 5: Synthesis of azasteroids using the Ugi-4CR with androstanic and pregnanic carboxylic acids [22].

Figure 2: Ugi-4CR-derived library of androstanic azasteroids with diverse substitution patterns at the phenyl...

Scheme 6: Synthesis of 4-azacholestanes by an intramolecular Ugi-4C-3R [26].

Scheme 7: Synthesis of amino acid–steroid hybrid by multiple Ugi-4CR using steroidal isocyanides [29].

Scheme 8: Synthesis of ecdysteroid derivatives by Ugi-4CR using a steroidal isocyanide [30].

Scheme 9: Stereoselective multicomponent synthesis of a steroid–tetrahydropyridine hybrid using a chiral bifu...

Scheme 10: Pd(II)-catalyzed three-component reaction with an alkynyl seco-cholestane [34].

Scheme 11: Multicomponent synthesis of steroid–thiazole hybrids from a steroidal ketone [36].

Scheme 12: Synthesis of cholanic pseudo-peptide derivatives by novel MCRs based on the reactivity of ynamide [37,38].

Scheme 13: Synthesis of steroid-fused pyrimidines and pyrimidones using the Biginelli-3CR [39,42,43].

Scheme 14: Synthesis of steroidal pyridopyrimidines by a reaction sequence comprising a 4CR followed by a post...

Scheme 15: Synthesis of steroid-fused pyrimidines by MCR of 2-hydroxymethylene-3-ketosteroids [46].

Scheme 16: Synthesis of steroid-fused naphthoquinolines by the Kozlov–Wang MCR using ketosteroids [50,51].

Scheme 17: Conjugation of steroids to carbohydrates and peptides by the Ugi-4CR [62,63].

Scheme 18: Solid-phase multicomponent conjugation of peptides to steroids by the Ugi-4CR [64].

Scheme 19: Solid-phase multicomponent conjugation of peptides to steroids by the Petasis-3CR [68].

Scheme 20: Synthesis of steroidal macrobicycles (cages) by multiple multicomponent macrocyclizations based on ...

Scheme 21: One-pot synthesis of steroidal cages by double Ugi-4CR-based macrocyclizations [76].
An efficient and concise access to 2-amino-4H-benzothiopyran-4-one derivatives
- Peng Li,
- Yongqi Wu,
- Tingting Zhang,
- Chen Ma,
- Ziyun Lin,
- Gang Li and
- Haihong Huang
Beilstein J. Org. Chem. 2019, 15, 703–709, doi:10.3762/bjoc.15.65
- from 2’-chloroacetophenone as the starting material through treatment with carbon disulfide in the presence of sodium hydride [23], followed by alkylation using iodoethane according to the literature procedures [13][16][17][19]. The oxidation of 1 with 1.2 or 5 equiv of H2O2 yielded ethyl sulfoxide 2

Graphical Abstract

Scheme 1: Representative strategies for the synthesis of N-substituted 2-aminobenzothiopyranones.

Scheme 2: The synthesis of sulfide 1, sulfoxide 2, and sulfone 3.

Scheme 3: Scope of the synthesis of versatile 2-aminobenzothiopyranones. All reactions were performed with 1....

Scheme 4: The gram-scale synthesis of 2-aminobenzothiopyranones 4a and 4d.
Metal-free C–H mercaptalization of benzothiazoles and benzoxazoles using 1,3-propanedithiol as thiol source
- Yan Xiao,
- Bing Jing,
- Xiaoxia Liu,
- Hongyu Xue and
- Yajun Liu
Beilstein J. Org. Chem. 2019, 15, 279–284, doi:10.3762/bjoc.15.24
- -catalyzed C–S coupling reactions [13]. Conventional methods for the synthesis of 2-mercaptobenzoxazoles and 2-mercaptobenzothiazoles include the interaction of 2-aminophenol or 2-haloanilines with carbon disulfide [14][15][16], or potassium ethyl xanthate [17][18] (Scheme 1). In 2017, the Dong group

Graphical Abstract

Figure 1: Representative examples of biologically active 2-mercaptobenzoxazoles and 2-mercaptobenzothiazoles.

Scheme 1: Strategies for the synthesis of 2-mercaptobenzothiazole and 2-mercaptobenzoxazole (X = O, S).

Figure 2: Substrate scope of the developed C–H mercaptalization strategy. Reaction conditions: benzothiazole ...

Scheme 2: Control experiments.

Scheme 3: Proposed reaction pathway.
5-Aminopyrazole as precursor in design and synthesis of fused pyrazoloazines
- Ranjana Aggarwal and
- Suresh Kumar
Beilstein J. Org. Chem. 2018, 14, 203–242, doi:10.3762/bjoc.14.15
- chlorides, chloroacetyl chloride, isothiocyanate and carbon disulfide under appropriate reaction conditions (Scheme 52). Hassan et al. [130] reported the synthesis of various pyrazolo[3,4-d]pyrimidine derivatives 193 (Scheme 53). 5-Aminopyrazole-4-carboxylic acid 190 on refluxing in acetic anhydride

Graphical Abstract

Figure 1: Selected examples of drugs with fused pyrazole rings.

Figure 2: Typical structures of some fused pyrazoloazines from 5-aminopyrazoles.

Scheme 1: Regiospecific synthesis of 4 and 6-trifluoromethyl-1H-pyrazolo[3,4-b]pyridines.

Scheme 2: Synthesis of pyrazolo[3,4-b]pyridine-6-carboxylates.

Scheme 3: Synthesis of 1,4,6-triaryl-1H-pyrazolo[3,4-b]pyridines with ionic liquid .

Scheme 4: Synthesis of coumarin-based isomeric tetracyclic pyrazolo[3,4-b]pyridines.

Scheme 5: Synthesis of 6-substituted pyrazolo[3,4-b]pyridines under Heck conditions.

Scheme 6: Microwave-assisted palladium-catalyzed synthesis of pyrazolo[3,4-b]pyridines.

Scheme 7: Acid-catalyzed synthesis of pyrazolo[3,4-b]pyridines via enaminones.

Scheme 8: Synthesis of pyrazolo[3,4-b]pyridines via aza-Diels–Alder reaction.

Scheme 9: Synthesis of macrocyclane fused pyrazolo[3,4-b]pyridine derivatives.

Scheme 10: Three-component synthesis of 4,7-dihydro-1H-pyrazolo[3,4-b]pyridine derivatives.

Scheme 11: Ultrasonicated synthesis of spiro[indoline-3,4'-pyrazolo[3,4-b]pyridine]-2,6'(1'H)-diones.

Scheme 12: Synthesis of spiro[indoline-3,4'-pyrazolo[3,4-b]pyridine] derivatives under conventional heating co...

Scheme 13: Nanoparticle-catalyzed synthesis of pyrazolo[3,4-b]pyridine-spiroindolinones.

Scheme 14: Microwave-assisted multicomponent synthesis of spiropyrazolo[3,4-b]pyridines.

Scheme 15: Unexpected synthesis of naphthoic acid-substituted pyrazolo[3,4-b]pyridines.

Scheme 16: Multicomponent synthesis of variously substituted pyrazolo[3,4-b]pyridine derivatives.

Scheme 17: Three-component synthesis of 4,7-dihydropyrazolo[3,4-b]pyridines and pyrazolo[3,4-b]pyridines.

Scheme 18: Synthesis of pyrazolo[3,4-b]pyridine-5-spirocycloalkanediones.

Scheme 19: Ultrasound-mediated three-component synthesis of pyrazolo[3,4-b]pyridines.

Scheme 20: Multicomponent synthesis of 4-aryl-3-methyl-1-phenyl-4,6,8,9-tetrahydropyrazolo [3,4-b]thiopyrano[4...

Scheme 21: Synthesis of 2,3-dihydrochromeno[4,3-d]pyrazolo[3,4-b]pyridine-1,6-diones.

Scheme 22: FeCl3-catalyzed synthesis of o-hydroxyphenylpyrazolo[3,4-b]pyridine derivatives.

Scheme 23: Ionic liquid-mediated synthesis of pyrazolo[3,4-b]pyridines.

Scheme 24: Microwave-assisted synthesis of pyrazolo[3,4-b]pyridines.

Scheme 25: Multicomponent synthesis of pyrazolo[3,4-b]pyridine-5-carbonitriles.

Scheme 26: Unusual domino synthesis of 4,7-dihydropyrazolo[3,4-b]pyridine-5-nitriles.

Scheme 27: Synthesis of 4,5,6,7-tetrahydro-4H-pyrazolo[3,4-b]pyridines under conventional heating and ultrasou...

Scheme 28: L-Proline-catalyzed synthesis of of pyrazolo[3,4-b]pyridine.

Scheme 29: Microwave-assisted synthesis of 5-aminoarylpyrazolo[3,4-b]pyridines.

Scheme 30: Microwave-assisted multi-component synthesis of pyrazolo[3,4-e]indolizines.

Scheme 31: Synthesis of fluoropropynyl and fluoroalkyl substituted pyrazolo[1,5-a]pyrimidine.

Scheme 32: Acid-catalyzed synthesis of pyrazolo[1,5-a]pyrimidine derivatives.

Scheme 33: Chemoselective and regiospecific synthesis of 2-(3-methylpyrazol-1’-yl)-5-methylpyrazolo[1,5-a]pyri...

Scheme 34: Regioselective synthesis of 7-trifluoromethylpyrazolo[1,5-a]pyrimidines.

Scheme 35: Microwave-assisted synthesis of 7-trifluoromethylpyrazolo[1,5-a]pyrimidine carboxylates.

Scheme 36: Microwave and ultrasound-assisted synthesis of 7-trifluoromethylpyrazolo[1,5-a]pyrimidines.

Scheme 37: Base-catalyzed unprecedented synthesis of pyrazolo[1,5-a]pyrimidines via C–C bond cleavage.

Scheme 38: Synthesis of aminobenzothiazole/piperazine linked pyrazolo[1,5-a]pyrimidines.

Scheme 39: Synthesis of aminoalkylpyrazolo[1,5-a]pyrimidine-7-amines.

Scheme 40: Synthesis of pyrazolo[1,5-a]pyrimidines from condensation of 5-aminopyrazole 126 and ethyl acetoace...

Scheme 41: Synthesis of 7-aminopyrazolo[1,5-a]pyrimidines.

Scheme 42: Unexpected synthesis of 7-aminopyrazolo[1,5-a]pyrimidines under solvent free and solvent-mediated c...

Scheme 43: Synthesis of N-(4-aminophenyl)-7-aryloxypyrazolo[1,5-a]pyrimidin-5-amines.

Scheme 44: Base-catalyzed synthesis of 5,7-diarylpyrazolo[1,5-a]pyrimidines.

Scheme 45: Synthesis of 6,7-dihydropyrazolo[1,5-a]pyrimidines in PEG-400.

Scheme 46: Synthesis of 7-heteroarylpyrazolo[1,5-a]pyrimidine-3-carboxamides.

Scheme 47: Synthesis of 7-heteroarylpyrazolo[1,5-a]pyrimidine derivatives under conventional heating and micro...

Scheme 48: Synthesis of N-aroylpyrazolo[1,5-a]pyrimidine-5-amines.

Scheme 49: Regioselective synthesis of ethyl pyrazolo[1,5-a]pyrimidine-7-carboxylate.

Scheme 50: Sodium methoxide-catalyzed synthesis of 3-cyano-6,7-diarylpyrazolo[1,5-a]pyrimidines.

Scheme 51: Synthesis of various pyrazolo[3,4-d]pyrimidine derivatives.

Scheme 52: Synthesis of hydrazinopyrazolo[3,4-d]pyrimidine derivatives.

Scheme 53: Synthesis of N-arylidinepyrazolo[3,4-d]pyrimidin-5-amines.

Scheme 54: Synthesis of pyrazolo[3,4-d]pyrimidinyl-4-amines.

Scheme 55: Iodine-catalyzed synthesis of pyrazolo[3,4-d]pyrimidinones.

Scheme 56: Synthesis of ethyl 6-amino-2H-pyrazolo[3,4-d]pyrimidine-4-carboxylate.

Scheme 57: Synthesis of 4-substituted-(3,6-dihydropyran-4-yl)-1H-pyrazolo[3,4-d]pyrimidines.

Scheme 58: Synthesis of 1-(2,4-dichlorophenyl)pyrazolo[3,4-d]pyrimidin-4-yl carboxamides.

Scheme 59: Synthesis of 5-(1,3,4-thidiazol-2-yl)pyrazolo[3,4-d]pyrimidine.

Scheme 60: One pot POCl3-catalyzed synthesis of 1-arylpyrazolo[3,4-d]pyrimidin-4-ones.

Scheme 61: Synthesis of 4-amino-N1,C3-dialkylpyrazolo[3,4-d]pyrimidines under Suzuki conditions.

Scheme 62: Microwave-assisted synthesis of pyrazolo[3,4-b]pyrazines.

Scheme 63: Synthesis and derivatization of pyrazolo[3,4-b]pyrazine-5-carbonitriles.

Scheme 64: Synthesis of 2-thioxo-pyrazolo[1,5-a][1,3,5]triazin-4-ones.

Scheme 65: Synthesis of 2,3-dihydropyrazolo[1,5-a][1,3,5]triazin-4(1H)-one.

Scheme 66: Synthesis of pyrazolo[1,5-a][1,3,5]triazine-8-carboxylic acid ethyl ester.

Scheme 67: Microwave-assisted synthesis of 4,7-dihetarylpyrazolo[1,5-a][1,3,5]triazines.

Scheme 68: Alternative synthetic route to 4,7-diheteroarylpyrazolo[1,5-a][1,3,5]triazines.

Scheme 69: Synthesis of 4-aryl-2-ethylthio-7-methylpyrazolo[1,5-a][1,3,5]triazines.

Scheme 70: Microwave-assisted synthesis of 4-aminopyrazolo[1,5-a][1,3,5]triazine.

Scheme 71: Synthesis of pyrazolo[3,4-d][1,2,3]triazines from pyrazol-5-yl diazonium salts.

Scheme 72: Synthesis of 2,5-dihydropyrazolo[3,4-e][1,2,4]triazines.

Scheme 73: Synthesis of pyrazolo[5,1-c][1,2,4]triazines via diazopyrazolylenaminones.

Scheme 74: Synthesis of pyrazolo[5,1-c][1,2,4]triazines in presence of sodium acetate.

Scheme 75: Synthesis of various 7-diazopyrazolo[5,1-c][1,2,4]triazine derivatives.

Scheme 76: One pot synthesis of pyrazolo[5,1-c][1,2,4]triazines.

Scheme 77: Synthesis of 4-amino-3,7,8-trinitropyrazolo-[5,1-c][1,2,4]triazines.

Scheme 78: Synthesis of tricyclic pyrazolo[5,1-c][1,2,4]triazines by azocoupling reaction.
Photocatalytic formation of carbon–sulfur bonds
- Alexander Wimmer and
- Burkhard König
Beilstein J. Org. Chem. 2018, 14, 54–83, doi:10.3762/bjoc.14.4
- . Carbon disulfide Formation of 1,3-oxathiolane-2-thiones In 2016, Yadav and co-workers reported a photoredox-catalyzed C–S bond formation with carbon disulfide (CS2) as starting material (Scheme 30) [65]. The reaction of CS2 with styrenes under visible-light irradiation and Eosin Y as organic
- over the most important visible-light induced and photoredox-catalyzed approaches for the formation of C–S bonds (Scheme 1). The survey is structured according to the sulfur-containing starting material, beginning with sulfur at its lowest oxidation state – and covers: Thiols, thiocyanates, carbon
- disulfide, thioamide derivatives and sulfides Disulfides and thiosulfates Sulfoxides Sulfinic acids, sulfinate salts and sulfinamides Sulfonyl halides, sulfonyl hydrazines, thionyl chloride and sulfur dioxide C–S bond formations initiated by irradiation with light of wavelengths shorter than 380 nm or by

Graphical Abstract

Scheme 1: General overview over the sulfur-based substrates and reactive intermediates that are discussed in ...

Scheme 2: Photoredox-catalyzed radical thiol–ene reaction, applying [Ru(bpz)3](PF6)2 as photocatalyst.

Scheme 3: Photoredox-catalyzed thiol–ene reaction of aliphatic thiols with alkenes enabled by aniline derivat...

Scheme 4: Photoredox-catalyzed radical thiol–ene reaction for the postfunctionalization of polymers (a) and n...

Scheme 5: Photoredox-catalyzed thiol–ene reaction enabled by bromotrichloromethane as redox additive.

Scheme 6: Photoredox-catalyzed preparation of β-ketosulfoxides with Eosin Y as organic dye as photoredox cata...

Scheme 7: Greaney’s photocatalytic radical thiol–ene reaction, applying TiO2 nanoparticles as photocatalyst.

Scheme 8: Fadeyi’s photocatalytic radical thiol–ene reaction, applying Bi2O3 as photocatalyst.

Scheme 9: Ananikov’s photocatalytic radical thiol-yne reaction, applying Eosin Y as photocatalyst.

Scheme 10: Organocatalytic visible-light photoinitiated thiol–ene coupling, applying phenylglyoxylic acid as o...

Scheme 11: Xia’s photoredox-catalyzed synthesis of 2,3-disubstituted benzothiophenes, applying 9-mesityl-10-me...

Scheme 12: Wang’s metal-free photoredox-catalyzed radical thiol–ene reaction, applying 9-mesityl-10-methylacri...

Scheme 13: Visible-light benzophenone-catalyzed metal- and oxidant-free radical thiol–ene reaction.

Scheme 14: Visible-light catalyzed C-3 sulfenylation of indole derivatives using Rose Bengal as organic dye.

Scheme 15: Photocatalyzed radical thiol–ene reaction and subsequent aerobic sulfide-oxidation with Rose Bengal...

Scheme 16: Photoredox-catalyzed synthesis of diaryl sulfides.

Scheme 17: Photocatalytic cross-coupling of aryl thiols with aryl diazonium salts, using Eosin Y as photoredox...

Scheme 18: Photocatalyzed cross-coupling of aryl diazonium salts with cysteines in batch and in a microphotore...

Scheme 19: Fu’s [Ir]-catalyzed photoredox arylation of aryl thiols with aryl halides.

Scheme 20: Fu’s photoredox-catalyzed difluoromethylation of aryl thiols.

Scheme 21: C–S cross-coupling of thiols with aryl iodides via [Ir]-photoredox and [Ni]-dual-catalysis.

Scheme 22: C–S cross-coupling of thiols with aryl bromides, applying 3,7-bis-(biphenyl-4-yl)-10-(1-naphthyl)ph...

Scheme 23: Collin’s photochemical dual-catalytic cross-coupling of thiols with bromoalkynes.

Scheme 24: Visible-light-promoted C–S cross-coupling via intermolecular electron donor–acceptor complex format...

Scheme 25: Li’s visible-light photoredox-catalyzed thiocyanation of indole derivatives with Rose Bengal as pho...

Scheme 26: Hajra’s visible-light photoredox-catalyzed thiocyanation of imidazoheterocycles with Eosin Y as pho...

Scheme 27: Wang’s photoredox-catalyzed thiocyanation reaction of indoles, applying heterogeneous TiO2/MoS2 nan...

Scheme 28: Yadav’s photoredox-catalyzed α-C(sp3)–H thiocyanation reaction for tertiary amines, applying Eosin ...

Scheme 29: Yadav’s photoredox-catalyzed synthesis of 5-aryl-2-imino-1,3-oxathiolanes.

Scheme 30: Yadav’s photoredox-catalyzed synthesis of 1,3-oxathiolane-2-thiones.

Scheme 31: Li’s photoredox catalysis for the preparation of 2-substituted benzothiazoles, applying [Ru(bpy)3](...

Scheme 32: Lei’s external oxidant-free synthesis of 2-substituted benzothiazoles by merging photoredox and tra...

Scheme 33: Metal-free photocatalyzed synthesis of 2-aminobenzothiazoles, applying Eosin Y as photocatalyst.

Scheme 34: Metal-free photocatalyzed synthesis of 1,3,4-thiadiazoles, using Eosin Y as photocatalyst.

Scheme 35: Visible-light photoredox-catalyzed preparation of benzothiophenes with Eosin Y.

Scheme 36: Visible-light-induced KOH/DMSO superbase-promoted preparation of benzothiophenes.

Scheme 37: Jacobi von Wangelin’s photocatalytic approach for the synthesis of aryl sulfides, applying Eosin Y ...

Scheme 38: Visible-light photosensitized α-C(sp3)–H thiolation of aliphatic ethers.

Scheme 39: Visible-light photocatalyzed cross-coupling of alkyl and aryl thiosulfates with aryl diazonium salt...

Scheme 40: Visible-light photocatalyzed, controllable sulfenylation and sulfoxidation with organic thiosulfate...

Scheme 41: Rastogi’s photoredox-catalyzed methylsulfoxidation of aryl diazonium salts, using [Ru(bpy)3]Cl2 as ...

Scheme 42: a) Visible-light metal-free Eosin Y-catalyzed procedure for the preparation of vinyl sulfones from ...

Scheme 43: Visible-light photocatalyzed cross-coupling of sodium sulfinates with secondary enamides.

Scheme 44: Wang’s photocatalyzed oxidative cyclization of phenyl propiolates with sulfinic acids, applying Eos...

Scheme 45: Lei’s sacrificial oxidant-free synthesis of allyl sulfones by merging photoredox and transition met...

Scheme 46: Photocatalyzed Markovnikov-selective radical/radical cross-coupling of aryl sulfinic acids and term...

Scheme 47: Visible-light Eosin Y induced cross-coupling of aryl sulfinic acids and styrene derivatives, afford...

Scheme 48: Photoredox-catalyzed bicyclization of 1,7-enynes with sulfinic acids, applying Eosin Y as photocata...

Scheme 49: Visible-light-accelerated C–H-sulfinylation of arenes and heteroarenes.

Scheme 50: Visible-light photoredox-catalyzed β-selenosulfonylation of electron-rich olefins, applying [Ru(bpy)...

Scheme 51: Photocatalyzed preparation of β-chlorosulfones from the respective olefins and p-toluenesulfonyl ch...

Scheme 52: a) Photocatalyzed preparation of β-amidovinyl sulfones from sulfonyl chlorides. b) Preparation of β...

Scheme 53: Visible-light photocatalyzed sulfonylation of aliphatic tertiary amines, applying [Ru(bpy)3](PF6)2 ...

Scheme 54: Reiser’s visible-light photoredox-catalyzed preparation of β-hydroxysulfones from sulfonyl chloride...

Scheme 55: a) Sun’s visible-light-catalyzed approach for the preparation of isoquinolinonediones, applying [fac...

Scheme 56: Visible-light photocatalyzed sulfonylation/cyclization of vinyl azides, applying [Ru(bpy)3]Cl2 as p...

Scheme 57: Visible-light photocatalyzed procedure for the formation of β-ketosulfones from aryl sulfonyl chlor...

Scheme 58: Zheng’s method for the sulfenylation of indole derivatives, applying sulfonyl chlorides via visible...

Scheme 59: Cai’s visible-light induced synthesis of β-ketosulfones from sulfonyl hydrazines and alkynes.

Scheme 60: Photoredox-catalyzed approach for the preparation of vinyl sulfones from sulfonyl hydrazines and ci...

Scheme 61: Jacobi von Wangelin’s visible-light photocatalyzed chlorosulfonylation of anilines.

Scheme 62: Three-component photoredox-catalyzed synthesis of N-amino sulfonamides, applying PDI as organic dye....

Scheme 63: Visible-light induced preparation of complex sulfones from oximes, silyl enol ethers and SO2.
One-pot three-component route for the synthesis of S-trifluoromethyl dithiocarbamates using Togni’s reagent
- Azim Ziyaei Halimehjani,
- Martin Dračínský and
- Petr Beier
Beilstein J. Org. Chem. 2017, 13, 2502–2508, doi:10.3762/bjoc.13.247
- /bjoc.13.247 Abstract A one-pot three-component route for the synthesis of S-trifluoromethyl dithiocarbamates by the reaction of secondary amines, carbon disulfide and Togni’s reagent is described. The reactions proceed in moderate to good yields. A similar reaction using a primary aliphatic amine

Graphical Abstract

Scheme 1: Synthetic routes for the preparation of trifluoromethyl dithiocarbamates.

Scheme 2: Synthesis of S-trifluoromethyl dithiocarbamates. Isolated yields are given in parentheses.

Scheme 3: Formation of benzyl isothiocyanate in a reaction with benzylamine.

Figure 1: Variable temperature 1H NMR spectra of compound 4c (CH2 region on the left and CH3 region on the ri...

Figure 2: The Eyring plot obtained for the rotation around the N–C bond in compound 4c.

Figure 3: The optimized structure of compound 4b (left) and the transition state structure for the rotation a...
Mechanochemical synthesis of thioureas, ureas and guanidines
- Vjekoslav Štrukil
Beilstein J. Org. Chem. 2017, 13, 1828–1849, doi:10.3762/bjoc.13.178

- structure in a one-pot two-step mechanochemical sequence. Another typical synthetic method for the preparation of thioureas, particularly if the desired isothiocyanate is not available, is the condensation of an amine with carbon disulfide [36]. This reaction proceeds through the formation of a

Graphical Abstract

Scheme 1: a) Schematic representations of unsubstituted urea, thiourea and guanidine. b) Wöhler's synthesis o...

Figure 1: Antidiabetic (1–3) and antimalarial (4) drugs derived from ureas and guanidines currently available...

Scheme 2: The structures of some representative (thio)urea and guanidine organocatalysts 5–8 and anion sensor...

Scheme 3: Solid-state reactivity of isothiocyanates reported by Kaupp [30].

Scheme 4: a) Mechanochemical synthesis of aromatic and aliphatic di- and trisubstituted thioureas by click-co...

Figure 2: The supramolecular level of organization of thioureas in the solid-state.

Figure 3: The supramolecular level of organization of thioureas in the solid-state.

Scheme 5: Thiourea-based organocatalysts and anion sensors obtained by click-mechanochemical synthesis.

Scheme 6: Mechanochemical desymmetrization of ortho-phenylenediamine.

Scheme 7: Mechanochemical desymmetrization of para-phenylenediamine.

Scheme 8: a) Selected examples of a mechanochemical synthesis of aromatic isothiocyanates from anilines. b) O...

Scheme 9: In solution, aromatic N-thiocarbamoyl benzotriazoles 27 are unstable and decompose to isothiocyanat...

Scheme 10: Mechanosynthesis of a) bis-thiocarbamoyl benzotriazole 29 and b) benzimidazole thione 31. c) Synthe...

Figure 4: In situ Raman spectroscopy monitoring the synthesis of thiourea 28d in the solid-state. N-Thiocarba...

Scheme 11: a) The proposed synthesis of monosubstituted thioureas 32. b) Conversion of N-thiocarbamoyl benzotr...

Scheme 12: A few examples of mechanochemical amination of thiocarbamoyl benzotriazoles by in situ generated am...

Scheme 13: Mechanochemical synthesis of a) anion binding urea 33 by amine-isocyanate coupling and b) dialkylur...

Scheme 14: a) Solvent-free milling synthesis of the bis-urea anion sensor 35. b) Non-selective desymmetrizatio...

Scheme 15: a) HOMO−1 contours of mono-thiourea 19b and mono-urea 36. b) Mechanochemical synthesis of hybrid ur...

Scheme 16: Synthesis of ureido derivatives 38 and 39 from KOCN and hydrochloride salts of a) L-phenylalanine m...

Scheme 17: a) K2CO3-assisted synthesis of sulfonyl (thio)ureas. b) CuCl-catalyzed solid-state synthesis of sul...

Scheme 18: Two-step mechanochemical synthesis of the antidiabetic drug glibenclamide (2).

Scheme 19: Derivatization of saccharin by mechanochemical CuCl-catalyzed addition of isocyanates.

Scheme 20: a) Unsuccessful coupling of p-toluenesulfonamide and DCC in solution and by neat/LAG ball milling. ...

Scheme 21: a) Expansion of the saccharin ring by mechanochemical insertion of carbodiimides. b) Insertion of D...

Scheme 22: Synthesis of highly basic biguanides by ball milling.
Chemoselective synthesis of diaryl disulfides via a visible light-mediated coupling of arenediazonium tetrafluoroborates and CS2
- Jing Leng,
- Shi-Meng Wang and
- Hua-Li Qin
Beilstein J. Org. Chem. 2017, 13, 903–909, doi:10.3762/bjoc.13.91
- tetrafluoroborates; carbon disulfide; chemoselectivity; diaryl disulfides; photocatalyst; Findings The development of methods for the functionalization of peptides and proteins under mild conditions is a current frontier in the fields of chemistry, biology and drug discovery [1][2][3][4]. Most of the
- transformations with applications in industry is considered of high practical value. In this context, carbon disulfide, a cheap and abundant chemical, has been widely used as reactant and solvent in both industry and materials science. For example, Batanero and co-workers reported an electrochemical
- transformation of carbon disulfide into diaryl disulfides [11]. Sunlight as abundant and almost infinitely available energy resource has been widely used for chemical transformations in the sense of cost, safety, availability, and environmental friendliness [12][13][14][15]. Herein, we report a visible light

Graphical Abstract

Scheme 1: Chemoselective assembly of diaryl disulfides.

Scheme 2: A plausible reaction mechanism.
N-Propargylamines: versatile building blocks in the construction of thiazole cores
- S. Arshadi,
- E. Vessally,
- L. Edjlali,
- R. Hosseinzadeh-Khanmiri and
- E. Ghorbani-Kalhor
Beilstein J. Org. Chem. 2017, 13, 625–638, doi:10.3762/bjoc.13.61
- -propargylamines and carbon disulfide The first example of a cyclization of N-propargylamines 1 with carbon disulfide to lead to 5-methylenethiazolidine-2-thiones 2 was reported in 1949 by Batty and Weedon. The reaction took place in refluxing ethanol and generally afforded the corresponding products in good
- yields. It was also observed that products 2 rapidly formed by reaction of 1 with carbon disulfide in the presence of sodium hydroxide as the base in water at 20 °C. Further, the authors showed that treatment of methylene compound 2 with cold concentrated sulfuric acid gave the corresponding isomeric
- high yields through a condensation of commercially available and cheap N-propargylamine, allyl bromide, and carbon disulfide [100]. Conclusion Much work has been carried out during the past decade and has demonstrated that N-propargylamines are one of the most useful and versatile precursors in the

Graphical Abstract

Figure 1: Selected examples of bioactive thiazole derivatives.

Figure 2: Some natural sources of thiazoles.

Figure 3: Some important thiazole-based compounds derived from N-propargylamines.

Scheme 1: The synthesis of thiazole-2-thiones 3 through the thermal cyclocondensation of N-propargylamines 1 ...

Scheme 2: (a) One-pot synthesis of 2-benzylthiazolo[3,2-a]benzimidazoles 6 through a base-catalyzed cascade r...

Scheme 3: (a) Synthesis of 2-iminothiazolidines 11 from N-propargylamines 9 and isothiocyanates 10. (b) Synth...

Scheme 4: (a) Synthesis of 2-aminothiazoles 17 through the reaction of ethyl 4-aminobut-2-ynoate salts 15 wit...

Scheme 5: Synthesis of 5-(iodomethylene)-3-methylthiazolidines 27 described by Zhou.

Scheme 6: Mechanism that accounts for the formation of 27.

Scheme 7: Clausen’s synthesis of fluorescein thiazolidines 30.

Scheme 8: Synthesis of multiply substituted thiazolidines 33 from N-propargylamines 32 and blocked N-isothioc...

Scheme 9: (a) Microwave-assisted cyclization of N-propargyl thiocarbamate 34. (b) Synthesis of thiazoles 39 t...

Scheme 10: Synthesis of thiazolidines 42 (42’) from the reaction of β-oxodithioesters 40 (40’) with N-propargy...

Scheme 11: Synthesis of 5-(dibromomethyl)thiazoles 44 via halocyclization of N-propargylamines 43 described by...

Scheme 12: Synthesis of dihydrothiazoles 46 through the treatment of N-propargylamides 45 with Lawesson’s reag...

Scheme 13: Synthesis of thiazoles 49 by treatment of silyl-protected N-propargylamines 47 with benzotriazolylt...

Scheme 14: Mechanism proposed to explain the synthesis of 2,5-disubstituted thiazoles 49 developed by Sasmal.

Scheme 15: Mo-catalyzed cyclization of N-propargylthiocarbamate 50.

Scheme 16: (a) DABCO-mediated intramolecular cyclization of N-(propargylcarbamothioyl)amides 53 to the corresp...

Scheme 17: Proposed mechanism for the generation of the iodine-substituted 4H-1,3-thiazines 56 and 4,5-dihydro...

Scheme 18: Au(III)-catalyzed synthesis of 5-alkylidenedihydrothiazoles 58 developed by Stevens.
A novel method for heterocyclic amide–thioamide transformations
- Walid Fathalla,
- Ibrahim A. I. Ali and
- Pavel Pazdera
Beilstein J. Org. Chem. 2017, 13, 174–181, doi:10.3762/bjoc.13.20
- efficient method for the transformation of heterocyclic amides to heterocyclic thioamides gained great attention. The reaction of three molar equivalents of cyclohexylamine (1) with one molar equivalent of carbon disulfide in water typically afforded N-cyclohexyl dithiocarbamate cyclohexylammonium salt (2
- cyclohexylamine (60 mmol) and water (50 mL) was added carbon disulfide (21 mmol) dropwise. The reaction mixture was stirred at room temperature for 2 h. The white solid obtained was filtered, washed with water, dried and crystalized from ethanol to provide the pure product. Yield 98% (ethanol 95%) white crystals

Graphical Abstract

Scheme 1: Synthesis of N-cyclohexyl dithiocarbamate cyclohexylammonium salt (2).

Scheme 2: The two-step thiation of quinazolin-4-one A1–6 and phthalazin-1-ones A7 and A8.

Scheme 3: Thiation of quinoline A9 and quinoxalinone A10–13.

Scheme 4: Rational mechanism of the reaction of 4-chloro-2-phenylquinazoline (B2) to 2-phenylquinazolin-4(3H)...
[2.2]Paracyclophane derivatives containing tetrathiafulvalene moieties
- Laura G. Sarbu,
- Lucian G. Bahrin,
- Peter G. Jones,
- Lucian M. Birsa and
- Henning Hopf
Beilstein J. Org. Chem. 2015, 11, 1917–1921, doi:10.3762/bjoc.11.207
- dropwise at room temperature to a solution of one equivalent of ketone 1 in dioxane, providing 2-bromo-1-([2.2]paracyclophan-4-yl)ethan-1-one (2) in 81% yield. The reactions of α-bromophenones with salts of dithiocarbamic acid, readily available from the reaction of secondary amines with carbon disulfide

Graphical Abstract

Scheme 1: Synthesis of 2-N,N-dialkylamino-4-([2.2]paracyclophan-4-yl)-1,3-dithiol-2-ylium perchlorates 5.

Figure 1: Molecular structure of compound 4a. Ellipsoids represent 30% probability levels. Selected molecular...

Scheme 2: Synthesis of tetrathiafulvalenes 7.

Figure 2: Molecular structure of compound 6 (two independent molecules). Ellipsoids represent 30% probability...






